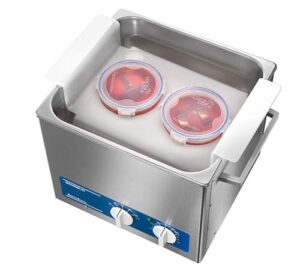
BactoSonic
Special ultrasonic bath for the gentle removal of biofilms

Compact & powerful
Kleines Ultraschallbad f├╝r gro├¤e Anwendungen
SweepTec
zur st├żndigen Schallfeldoszillation f├╝r gleichm├ż├¤ige Beschallung
Robust
The robust stainless steel housing is easy to clean and protected against dripping water
Power setting
from 20% to 100%
Low intensity, high homogeneity
Time, temperature, power and thus adaptation to the respective sample body
Level indicator
For safe and accurate filling of the unit
Working principle of the BactoSonic
Das Implantat wird in die Fl├╝ssigkeit der entsprechenden Box gelegt und in dem f├╝r dieses Verfahren speziell entwickelte BactoSonic Ultraschallbad sonifiziert. Dieses Ger├żt arbeitet im Vergleich zu anderen Ultraschallb├żdern mit niederfrequentem Ultraschall bei geringer Intensit├żt und hoher Homogenit├żt. Ziel ist die Entfernung des Biofilms, ohne die Bakterien zu zerst├Čren, die f├╝r die nachfolgende Analyse erhalten bleiben m├╝ssen. Die erhaltene Fl├╝ssigkeit wird mikrobiologisch verarbeitet und die Bakterienmenge quantitativ angegeben. Es k├Čnnen bis zu 10.000-mal mehr Bakterien nachgewiesen werden als mit ├╝blichen Methoden, wie zum Beispiel aus Biopsien von periprothetischem Gewebe. Mischinfektionen und unterschiedliche Bakterien-Morphotype k├Čnnen besser nachgewiesen werden. Die Sensitivit├żt ist insbesondere bei Patienten mit vorangegangener Antibiotikatherapie verbessert.
Technical details can be found in the Data sheet.
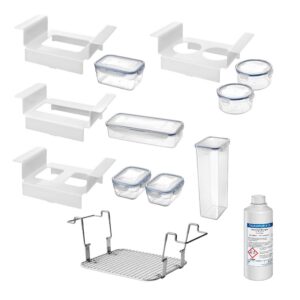
Ready-to-use set - order no. 3291
BactoSonic 14.2
Implants and infection
With the increasing use of medical implants, we are also confronted increasingly confronted with infectious biofilms on them. The most most common implants are joint prostheses, osteosyntheses, vascular prostheses, pacemakers and defibrillators, dental implants, neurosurgical shunts and breast implants.
The success of treatment for implant infections depends on a precise
precise, microbiological diagnosis. Because microorganisms form
foreign bodies, they are often difficult to detect in the surrounding tissue.
surrounding tissue.
Biofilms consist of an amorphous matrix of polymerised polysaccharide.
polymerised polysaccharide in which microorganisms are embedded.
are embedded. In the biofilm, microorganisms are in a metabolically less active
stationary growth phase. Over weeks to years
a complex three-dimensional layer develops, which, via rudimentary
the nutrition of the biofilm (via water channels) and its communication (via extracellular
and its communication (through extracellular messengers).
ensures.
While free-living (planktonic) bacteria are killed by antibiotics and the immune system, adherent immune system, adherent bacteria survive protected in the extra cellular in the extra cellular matrix of the biofilm.


Detection of biofilm-forming bacteria
The implant is aseptically removed from the body in the operating theatre and transported in a sterile box to the microbiology laboratory within 24 hours. After addition of Ringer's solution, the implant is shaken vigorously and exposed to ultrasound for 1 minute. The sonication removes > 99.9% of the adherent bacteria.
Quantitative detection of biofilm-forming bacteria


Detection of biofilm-forming bacteria
various
Implant boxes

Bandelin Electronic
Quality since 1955
We - a Berlin-based family business in its third generation - specialise in the development, manufacture and distribution of ultrasonic devices, corresponding accessories and application-specific cleaning and disinfection preparations. The high vertical range of manufacture, a modern production facility and motivated employees distinguish us and are guarantors for constantly new quality products.